
Most calcium salts dissolve readily in water.Around 97% of naturally occurring calcium is in the form of the isotope 40Ca. Two more calcium isotopes ( 46Ca and 48Ca) have very long half-lives and are considered mostly stable. Sir Humphry Davy named calcium after the Latin word "calx" which is what the Romans called lime.Ĭalcium has four stable isotopes including 40Ca, 42Ca, 43Ca, and 44Ca. The first scientist to discover and isolate the element calcium was English chemist Sir Humphry Davy in 1808. Calcium is the fifth most abundant element in the human body, making up around 1.4% of the body's mass. In the human body calcium is part of a compound called hydroxyapatite which is what makes our bones and teeth hard. Other applications include antacids, toothpaste, and fertilizer.Ĭalcium is also a very important element in both plant and animal life. Gypsum is used to make plaster of Paris and drywall. It is also used to produce additional chemicals.Ĭalcium compounds, rocks, and minerals such as limestone and marble are also used in construction. Lime is used in a number of applications including the production of metals, removing pollution, and water purification. One important compound is calcium oxide (CaO), which is also called lime. It is the fifth most common element in the Earth's crust.Ĭalcium carbonate is one of the major components of many rocks and minerals including limestone, marble, calcite, and chalk.Ĭalcium is also found in ocean water and is about the eighth most abundant element found in the ocean.Ĭalcium in its elemental form has few industrial uses, but its compounds with other elements are widely used. When burned, it produces a bright orange-red flame.Ĭalcium is rarely found in its elemental form, but is readily found throughout the Earth mostly in the form of rocks and minerals such as limestone (calcium carbonate), dolomite (calcium magnesium carbonate), and gypsum (calcium sulfate).

When exposed to water, calcium will react and generate hydrogen. Although it is a bright silver when first cut, it will quickly form a gray-white oxide on its surface when exposed to air. It is fairly soft and is the lightest of the alkaline earth metals due to its low density.

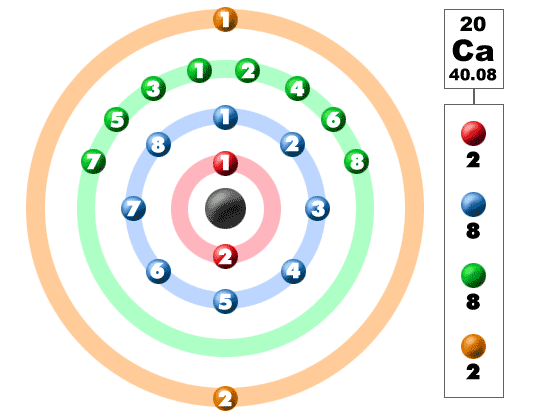
Under standard conditions calcium is a shiny, silvery metal. Calcium is an important element for life on Earth and is the fifth most abundant element in the Earth's crust. There are 2 valence electrons in the outer shell. Calcium atoms have 20 electrons and 20 protons. It is classified as an alkaline earth metal. Discovered by: Sir Humphry Davy in 1808Ĭalcium is the third element in the second column of the periodic table.


 0 kommentar(er)
0 kommentar(er)
